SCIENCE 30
Unit B: Chemistry and the Environment
Focusing Questions:
- What are some of the important effects of acids, bases and synthetic organic compounds on the environment and living systems?
- What are the chemical principles and perspectives involved in the assessment of the technologies designed to reduce the production and emission of these compounds into the environment?
- How does society look beyond a technological fix in deciding how to best meet human needs while sustaining the environment?
Chapter 2: The Chemical Legacy of Human Activity
General Outcome B2 - Students will analyze the sources of organic compounds and their effects on the environment.
30–B2.1k identify and name carbon compounds, using International Union of Pure and Applied Chemistry (IUPAC) nomenclature that contain up to three carbon atoms in the parent chain and a single occurrence of one type of functional group, including simple halogenated hydrocarbons (e.g., 2-chloropropane), alcohols (e.g., propan-1-ol), carboxylic acids (e.g., propanoic acid) and esters (e.g., methyl propanoate)30–B2.2k describe the common uses of hydrocarbons, including simple halogenated hydrocarbons, alcohols, carboxylic acids and esters; e.g., chlorofluorocarbons (CFCs) as refrigerants, as propellants and in the manufacture of plastic foam products; ethanol as a solvent and as a gasoline additive; ethanoic acid as vinegar; ethyl ethanoate as nail-polish remover
30–B2.3k identify organic compounds commonly considered to be environmental pollutants; i.e., hydrocarbons, organic waste, CFCs, polychlorinated biphenyls (PCBs), dioxins and furans
30–B2.4k list the sources of, and analyze the hazards posed by, halogenated hydrocarbons and benzene derivatives
30–B2.5k identify and explain how human activities and natural events contribute to the production of photochemical smog, the depletion of the ozone layer and increased concentrations of organic compounds in the environment; e.g., driving a car, use of CFCs, agricultural practices
30–B2.6k explain the mechanism and significance of biomagnification.
General Outcome B3 - Students will analyze, from a variety of perspective, the risks and benefits of using chemical processes in meeting human needs and assess technologies for reducing the impact of chemical compounds on the environment.
30–B3.1k describe the risks and benefits of using chemical processes that may produce products and/or by-products that have the potential to harm the environment30–B3.2k describe technologies used to reduce the production and emission of chemical compounds that have the potential to harm the environment; e.g., activities related to internal combustion engines, smelting, pesticide production, sweetening of sour gas
30–B3.3k describe alternatives to the use of chemical technologies; e.g., bioremediation for contaminated soil, biological controls for pests, biodegradable products.
2.1 Organic Compounds
Organic Compounds
- Organic compounds are based on long chains of carbon.
- Each carbon atom forms 4 bonds in a tetrahedral shape, but we often draw them as linear chains.
- Count the number of carbons and use the data booklet to determine the name.
Organic Molecule Maker Link *****************************
| Homologous Series of Alkanes at 25o C and 101.325 kPa | |||
| Name | formula | Name | Formula |
|---|---|---|---|
| methane | CH4 | hexane | C6H14 |
| ethane | C2H6 | heptane | C7H16 |
| propane | C3H8 | octane | C8H18 |
| butane | C4H10 | nonane | C9H20 |
| pentane | C5H12 | decane | C10H22 |
- All carbons have four valence electrons and spaces for four more bonds.
- Always make sure every carbon has four bonds!
| Name | Diagram |
|---|---|
| methane | 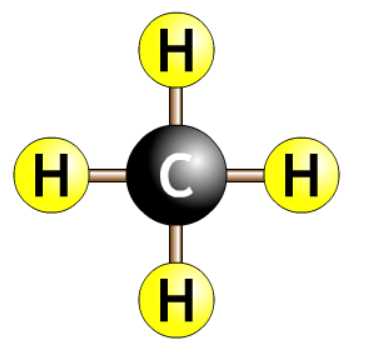 |
| ethane | 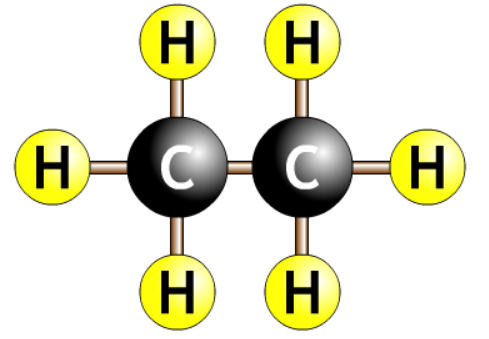 |
| propane | 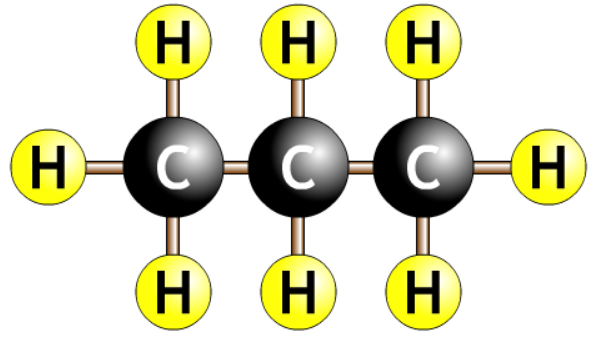 |
| butane | 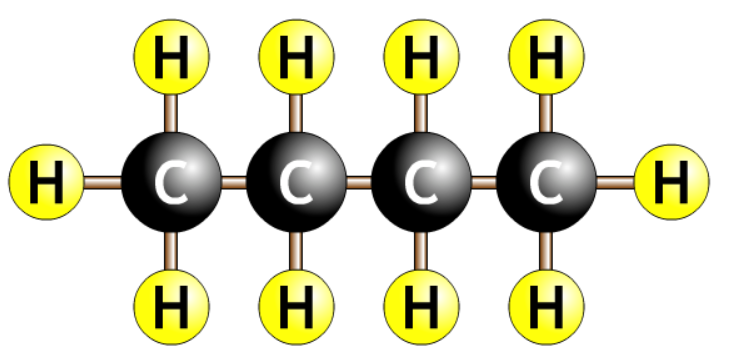 |
Alkanes
- Single bond carbon chain surrounded by hydrogens.
- The small chains as gases (1-4)
- The medium length chains are liquids (5-19)
- The long chains are solids (20+)
- We use the gases and liquids as fuels. Next time you fill up with gasoline check the pump. You will see octane written on it. Octane is an 8 carbon chain alkane. Gasoline is a combination of alkanes around and 8 carbon chain.
- Carbon chain with a double bond surrounded by hydrogens.
- The number tells you where the double bond is.
- The eneene tells you there is a double bond.
- The number before the ene tells you where the double bond is.
- Always count to get the smallest number. Should never start with a number more than half the length of the chain.
- For Science 30 don’t worry about the hydrogen positions. ie. same side missing or opposite sides missing.
| Name | Diagram |
|---|---|
| ethene | 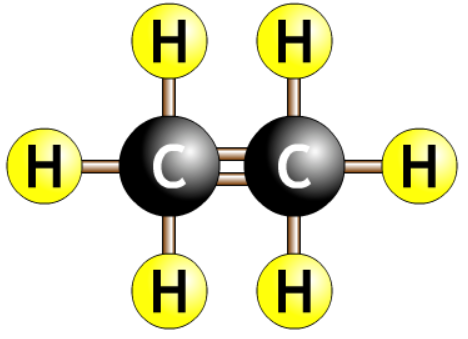 |
| prop-1-ene | 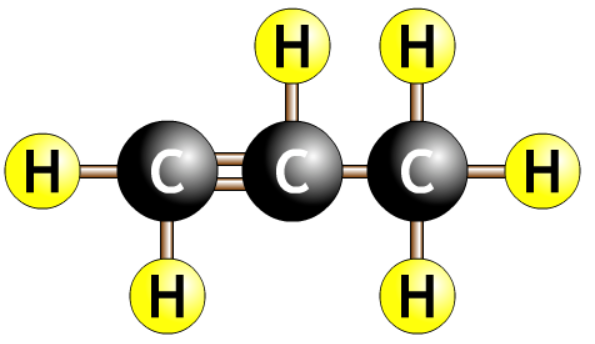 |
| prop-2-ene Should be prop-1-ene | 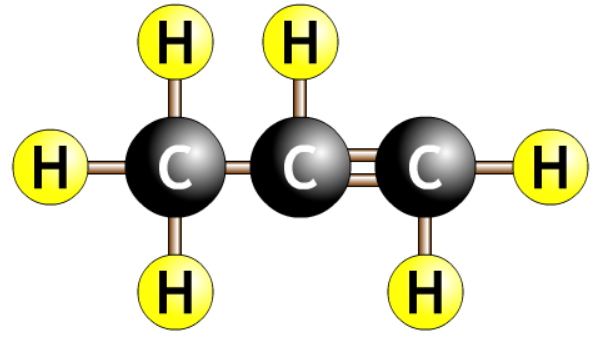 |
| prop-1,2-diene | 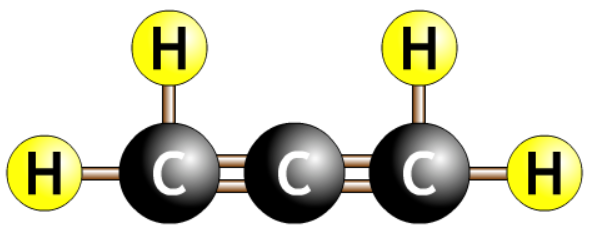 |
| but-1-ene | 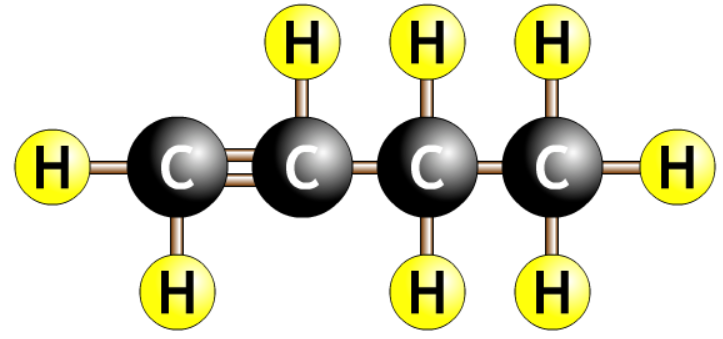 |
| but-2-ene | 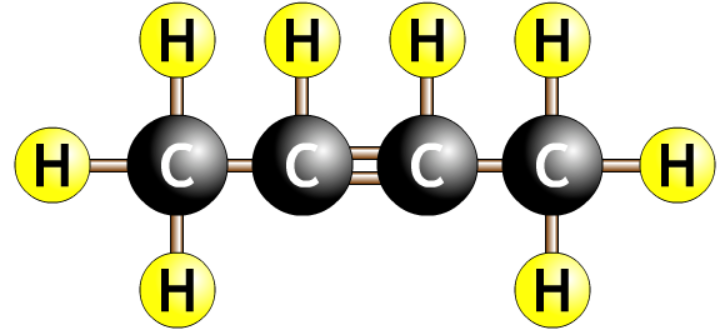 |
| but-3-ene Should be but-1-ene | 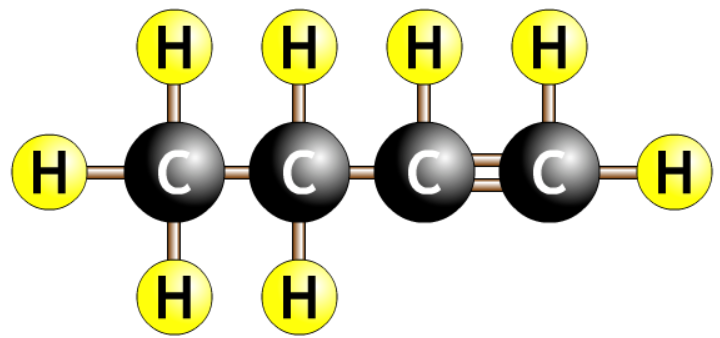 |
- 5 carbons - pent
- Double bonds on first (1), second (2), fourth (4) carbon
- Three double bonds - tri
- Double bond - ene
- Name: pent-1,2,4-tri-ene
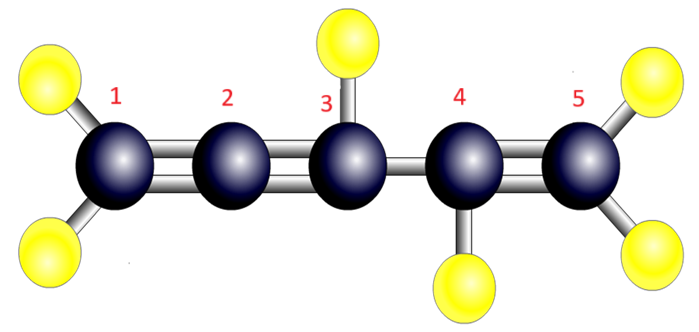
| Name | Diagram |
|---|---|
| ethene |  |
| prop-1-ene |  |
| prop-2-ene Should be prop-1-ene |  |
| prop-1,2-diene |  |
| but-1-ene |  |
| but-2-ene |  |
| but-3-ene Should be but-1-ene |  |
Alkynes
- Carbon chain with a triple bond surrounded by hydrogens.
- The number tells you where the triple bond is.
- The yne tells you there is a triple bond.
- The first part of the name tells you how long the carbon is.
- The number tells you where the triple bond is.
- The yne tells you there is a triple bond.
| Name | Diagram |
|---|---|
| ethyne | 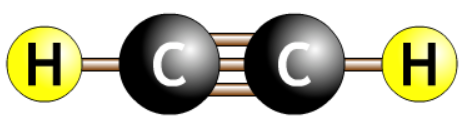 |
| prop-1-yne | 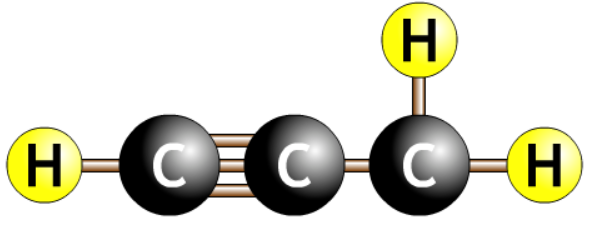 |
| prop-2-yne Should be prop-1-yne |  |
| but-1-yne | 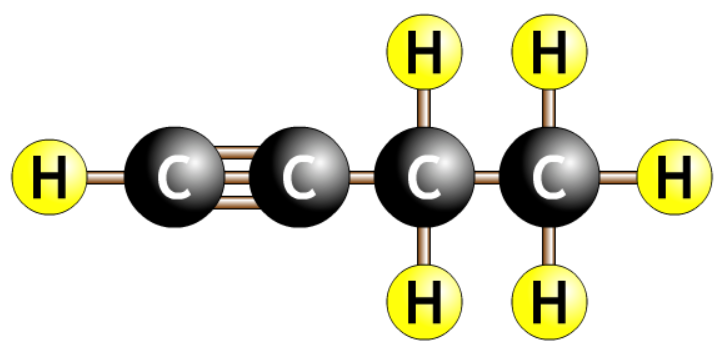 |
| but-2-yne | 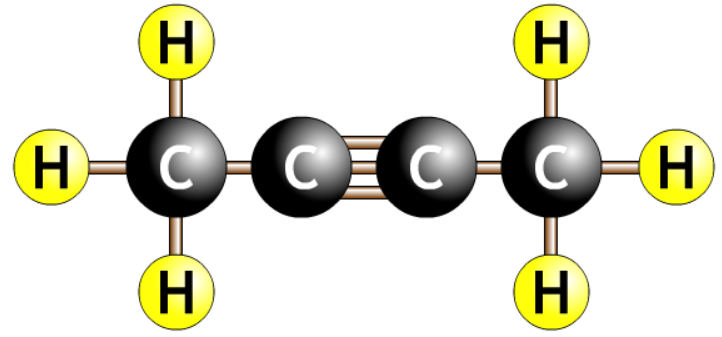 |
| but-3-yne Should be but-1-yne | 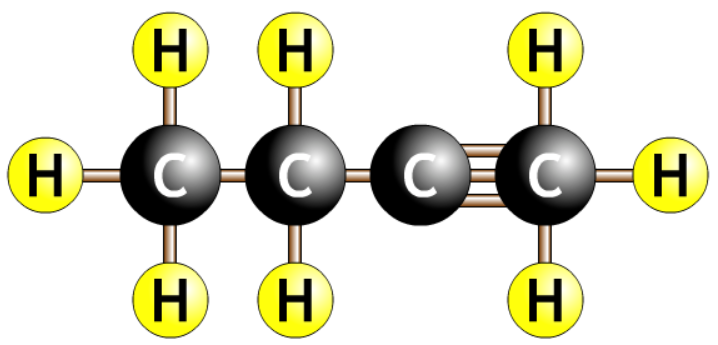 |
- End with a hydroxyl function group, OH
- The only “drinkable” alcohol is ethanol, others easily poison you.
| Name | Diagram |
|---|---|
| methanol | 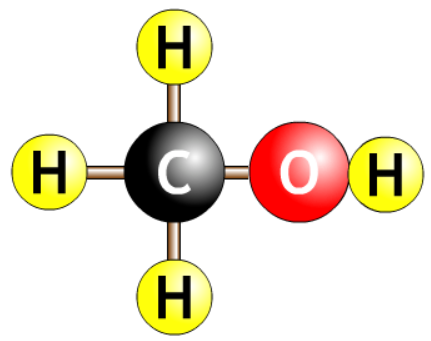 |
| ethanol | 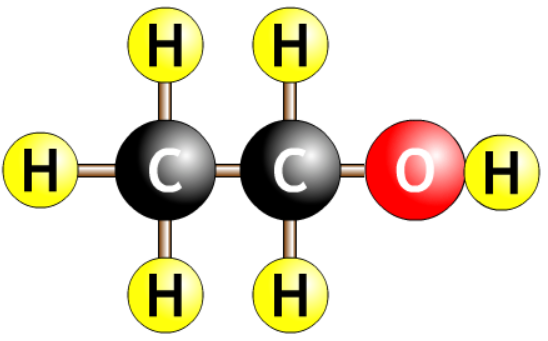 |
| propanol | 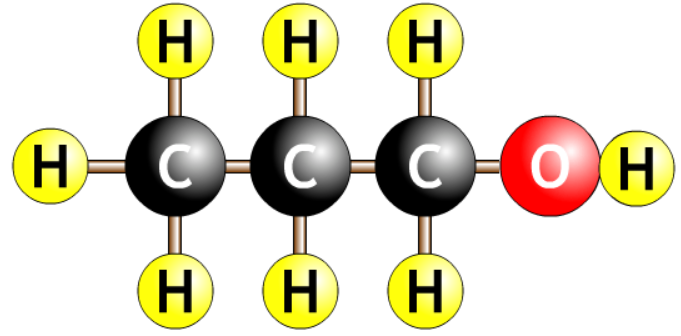 |
| butanol | 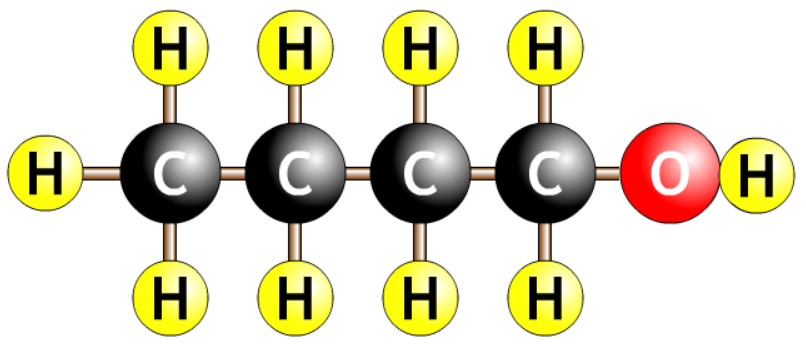 |
- Organic acids have a carboxyl group, COOH.
- Methanoic acid is in many insect stings.
- Some longer acids are plant oils, decanoic acid is palm oil.
| Name | Diagram |
|---|---|
| methanoic acid | 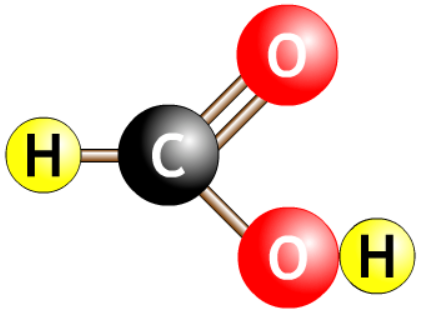 |
| ethanoic acid | 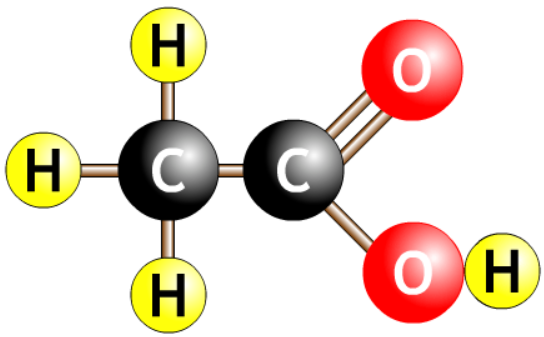 |
| propanoic acid | 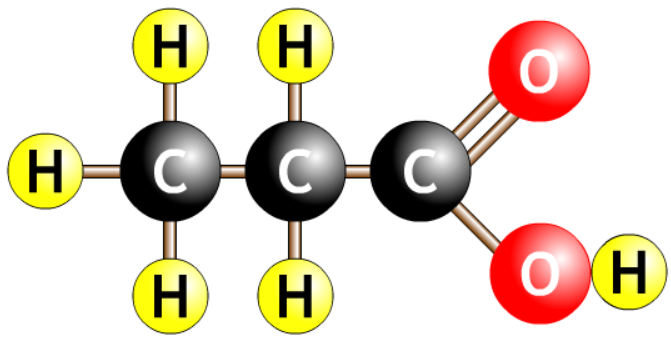 |
| butanoic acid | 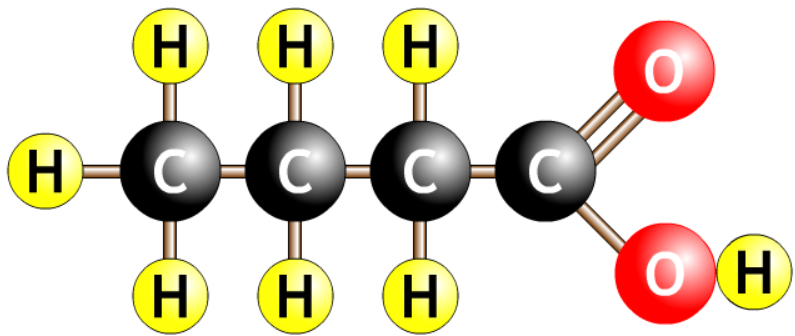 |
- A combination of a carboxylic acid and an alcohol.
- Propanoic acid and methanol form methyl propanoate.
- Esters often have strong smells.
| Name | Diagram |
|---|---|
| methyl methanoate | 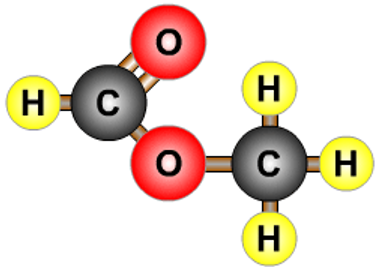 |
| methyl ethanoate | 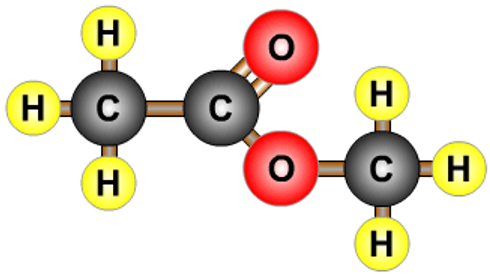 |
| methyl propanoate | 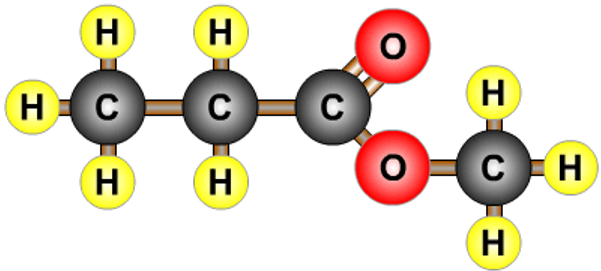 |
| methyl butanoate | 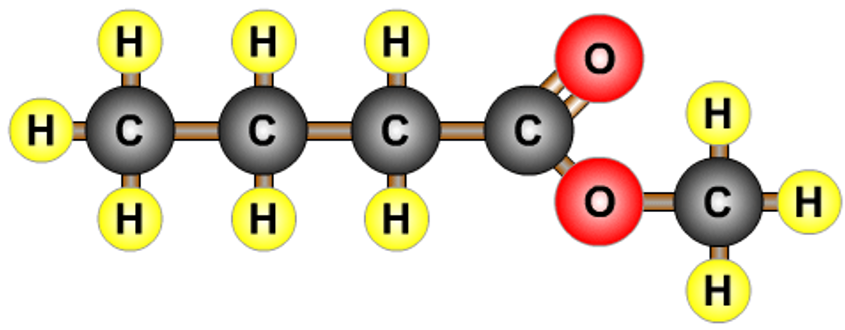 |
- When hydrogens are replaced by halogen elements.
-
Halogen Prefix bromine bromo chlorine chloro fluorine fluoro iodine iodo
- Ozone O3 is a form of oxygen that is found high in the atmosphere. It absorbs UV light breaking apart. It spontaneously recombines releasing the energy back into space.
- Chlorofluorocarbons (CFC) were used to push aerosols out of aerosol cans or used as a refrigerants. They were though to be inactive.
- Later in the 1970’s scientists realised that they interfered with the ozone layer. This was causing a hole (low density region) in the ozone layer, which let in more UV light over Antarctica.
- Internationally all countries agreed to significantly restrict the use of CFCs.
- Over time the ozone layer has been slowly recovering.
2.2 Alcohols, Carboxylic Acids, and Esters
Alcohols
- Written with OH to indicate the hydroxide functional end.
- Ethanol (beer, wine) is CH3CH2OH
- Industrially they are used as solvents (to dissolve chemicals).
- Written with COOH at the end of the formula to indicate the carboxyl functional end.
- Acetic acid (ethanoic acid, vinegar) is CH3COOH
- Written with OO in the middle to indicate the ester.
- Esters are made by combining an alcohol and a carboxylic acid.
carboxylic acid + alcohol → ester + water
The hydrogen in the OH of the alcohol combines with the OH from the acid to form water. - Ex.
carboxylic acid + alcohol → ester methanol + ethanoic acid → methyl ethanoate + water C3OH + CH3H2OOH → CH3OOCCH3 + H2O
2.3 Understanding Exposure
VOC
- Volatile organic compounds are “plastic” molecules that are released by off-gassing as plastics slowly finish reacting and forming.
- VOC have shown to increase the amount of plastic in humans. They were associated with “new car” smell, the smell of drying paints, and things that smell when new.
- VOC have been linked with health problems and sensitivities.
- Since 2010 many companies have worked extensively to ensure that they use products that produce less VOCs such as low VOC paints.
Targeting Toxic Chemicals
- Lots of substance are toxic to humans.
- Some require a lot of exposure, some only require a little to cause problems.
- Toxicity is measure as LD50 (lethal dose, single serving) or LC50 (leathal concentration, long term build up). That is the amount that is estimated to kill 50% of people who ingest that amount. It is often measure in the amount per kg of person. Larger people need more to be poisoned.
- The most dangerous chemicals are the ones that our body can’t get rid of. They slow build up our bodies until they kill us.
- Pesticides are a major concern as they are poisons designed to kill insects, but most are lethal to humans with enough exposure. Pesticides don’t just stay in the field, the wind can carry droplets long distance, weather can move the droplets long distance.
- Newer pesticides break down rapidly (organophosphates, 2-3 days) in sunlight, so they can’t travel as far before they break down.
- Fertilizers can also cause problems by promoting the growth of plants, whether humans want or not. Lots of lakes (Thunder lake) regularly suffer from excess algae due to fertilizer runoff.
- As algae die they consume oxygen as they decompose. If the oxygen levels in the water drop low enough fish and other plants will also die as a result.
- Biochemical (biological) oxygen demand (BOD) is a measure of how much oxygen things will consume as they decompose. It is also important around waste water treatment plants that discharge into water ways.